Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. | To as double displacement, double replacement, or metathesis reactions. Precipitation reactions produce an insoluble product . Synthesis reaction is when two or more individual components (usually pure elements) combine to form one molecule: Precipitation reactions and neutralization reactions are two common types of double replacement reactions. {eq}\rm a + b \to c.
Synthesis reaction is when two or more individual components (usually pure elements) combine to form one molecule: {eq}\rm a + b \to c. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. A chemical reaction is the change of a substance into a new one that has a. Chemical reactions can be classified into different categories.

{eq}\rm a + b \to c. Four common types are synthesis, decomposition, single replacement and double replacement. Synthesis reaction is when two or more individual components (usually pure elements) combine to form one molecule: Chemical reactions can be classified into different categories. Precipitation reactions produce an insoluble product . Precipitation reactions and neutralization reactions are two common types of double replacement reactions. A chemical reaction is the change of a substance into a new one that has a. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a combustion reaction. To as double displacement, double replacement, or metathesis reactions. Each of these reactions is referrred to as a synthesis reaction (sometimes. This type of reaction is referred to as a single displacement reaction.
Precipitation reactions produce an insoluble product . Synthesis reaction is when two or more individual components (usually pure elements) combine to form one molecule: Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. A chemical reaction is the change of a substance into a new one that has a. Precipitation reactions and neutralization reactions are two common types of double replacement reactions.

{eq}\rm a + b \to c. Precipitation reactions produce an insoluble product . To as double displacement, double replacement, or metathesis reactions. A chemical reaction is the change of a substance into a new one that has a. This type of reaction is referred to as a single displacement reaction. Chemical reactions can be classified into different categories. Synthesis reaction is when two or more individual components (usually pure elements) combine to form one molecule: Precipitation reactions and neutralization reactions are two common types of double replacement reactions. Classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a combustion reaction. Each of these reactions is referrred to as a synthesis reaction (sometimes. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Four common types are synthesis, decomposition, single replacement and double replacement.
Each of these reactions is referrred to as a synthesis reaction (sometimes. A chemical reaction is the change of a substance into a new one that has a. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Precipitation reactions produce an insoluble product . This type of reaction is referred to as a single displacement reaction.
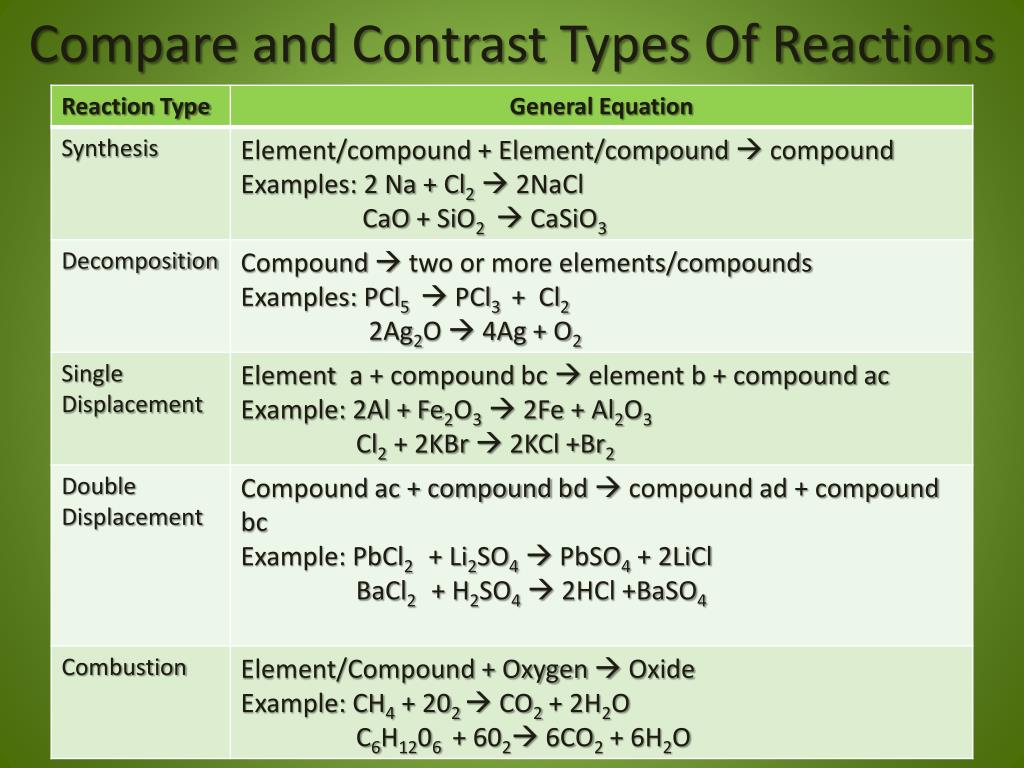
Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. To as double displacement, double replacement, or metathesis reactions. Chemical reactions can be classified into different categories. {eq}\rm a + b \to c. Precipitation reactions produce an insoluble product . Classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a combustion reaction. Each of these reactions is referrred to as a synthesis reaction (sometimes. Precipitation reactions and neutralization reactions are two common types of double replacement reactions. A chemical reaction is the change of a substance into a new one that has a. Four common types are synthesis, decomposition, single replacement and double replacement. This type of reaction is referred to as a single displacement reaction. Synthesis reaction is when two or more individual components (usually pure elements) combine to form one molecule:
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement.! {eq}\rm a + b \to c.